 What if CO2 could be captured and, rather than locked away underground for eternity, turned into a stable powder that can be used in fuel cells to produce electricity? David Chandler at MIT describes research there and at Harvard that has demonstrated a new process that has a 96% conversion rate. It’s been tried before, but the conversion rates were an unusable 20%. The CO2 is converted into formate and used like hydrogen or methanol (both strong candidates for future alternative fuel sources) to power a fuel cell. Unlike methanol it is non-toxic, and in solid form it can be stored for decades. It is produced at ambient temperatures and relatively low pressures (about five times atmospheric pressure). This is a lab-scale demonstration, but the researchers say the formate fuel can be adapted for anything from home-sized units to large scale industrial uses or grid-scale storage systems.
What if CO2 could be captured and, rather than locked away underground for eternity, turned into a stable powder that can be used in fuel cells to produce electricity? David Chandler at MIT describes research there and at Harvard that has demonstrated a new process that has a 96% conversion rate. It’s been tried before, but the conversion rates were an unusable 20%. The CO2 is converted into formate and used like hydrogen or methanol (both strong candidates for future alternative fuel sources) to power a fuel cell. Unlike methanol it is non-toxic, and in solid form it can be stored for decades. It is produced at ambient temperatures and relatively low pressures (about five times atmospheric pressure). This is a lab-scale demonstration, but the researchers say the formate fuel can be adapted for anything from home-sized units to large scale industrial uses or grid-scale storage systems.
Engineers develop an efficient process to make fuel from carbon dioxide. The approach directly converts the greenhouse gas into formate, a solid fuel that can be stored indefinitely and could be used to heat homes or power industries. By David Chandler, MIT News.
The search is on worldwide to find ways to extract carbon dioxide from the air or from power plant exhaust and then make it into something useful. One of the more promising ideas is to make it into a stable fuel that can replace fossil fuels in some applications. But most such conversion processes have had problems with low carbon efficiency, or they produce fuels that can be hard to handle, toxic, or flammable.
Turning CO2 into a liquid or solid fuel
Now, researchers at MIT and Harvard University have developed an efficient process that can convert carbon dioxide into formate, a liquid or solid material that can be used like hydrogen or methanol to power a fuel cell and generate electricity. Potassium or sodium formate, already produced at industrial scales and commonly used as a de-icer for roads and sidewalks, is nontoxic, nonflammable, easy to store and transport, and can remain stable in ordinary steel tanks to be used months, or even years, after its production.
The new process, developed by MIT doctoral students Zhen Zhang, Zhichu Ren, and Alexander H. Quinn; Harvard University doctoral student Dawei Xi; and MIT Professor Ju Li, is described in an open-access paper in Cell Reports Physical Science. The whole process — including capture and electrochemical conversion of the gas to a solid formate powder, which is then used in a fuel cell to produce electricity — was demonstrated at a small, laboratory scale. However, the researchers expect it to be scalable so that it could provide emissions-free heat and power to individual homes and even be used in industrial or grid-scale applications.
New process: conversion rate of 90%+
Other approaches to converting carbon dioxide into fuel, Li explains, usually involve a two-stage process: first the gas is chemically captured and turned into a solid form as calcium carbonate, then later that material is heated to drive off the carbon dioxide and convert it to a fuel feedstock such as carbon monoxide. That second step has very low efficiency, typically converting less than 20 percent of the gaseous carbon dioxide into the desired product, Li says.
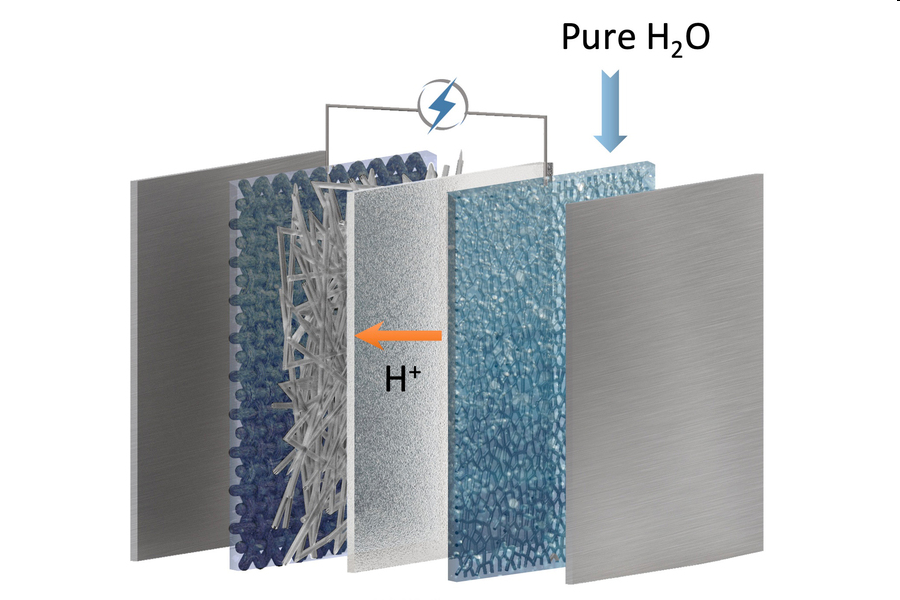
An electrolyser configuration with a bicarbonate cathode, intermediate buffer layer, cation exchange membrane and a water anode. / IMAGE: Shuhan Miao, Harvard Graduate School of Design
Solid powder that can last for decades
By contrast, the new process achieves a conversion of well over 90 percent and eliminates the need for the inefficient heating step by first converting the carbon dioxide into an intermediate form, liquid metal bicarbonate. That liquid is then electrochemically converted into liquid potassium or sodium formate in an electrolyser that uses low-carbon electricity, e.g. nuclear, wind, or solar power. The highly concentrated liquid potassium or sodium formate solution produced can then be dried, for example by solar evaporation, to produce a solid powder that is highly stable and can be stored in ordinary steel tanks for up to years or even decades, Li says.
Several steps of optimisation developed by the team made all the difference in changing an inefficient chemical-conversion process into a practical solution, says Li, who holds joint appointments in the departments of Nuclear Science and Engineering and of Materials Science and Engineering.
The process of carbon capture and conversion involves first an alkaline solution-based capture that concentrates carbon dioxide, either from concentrated streams such as from power plant emissions or from very low-concentration sources, even open air, into the form of a liquid metal-bicarbonate solution. Then, through the use of a cation-exchange membrane electrolyser, this bicarbonate is electrochemically converted into solid formate crystals with a carbon efficiency of greater than 96 percent, as confirmed in the team’s lab-scale experiments.
…easier to store than Methanol or Hydrogen
These crystals have an indefinite shelf life, remaining so stable that they could be stored for years, or even decades, with little or no loss. By comparison, even the best available practical hydrogen storage tanks allow the gas to leak out at a rate of about 1 percent per day, precluding any uses that would require year-long storage, Li says. Methanol, another widely explored alternative for converting carbon dioxide into a fuel usable in fuel cells, is a toxic substance that cannot easily be adapted to use in situations where leakage could pose a health hazard. Formate, on the other hand, is widely used and considered benign, according to national safety standards.
Achieving a pH balance
Several improvements account for the greatly improved efficiency of this process. First, a careful design of the membrane materials and their configuration overcomes a problem that previous attempts at such a system have encountered, where a buildup of certain chemical byproducts changes the pH, causing the system to steadily lose efficiency over time. “Traditionally, it is difficult to achieve long-term, stable, continuous conversion of the feedstocks,” Zhang says. “The key to our system is to achieve a pH balance for steady-state conversion.”
To achieve that, the researchers carried out thermodynamic modelling to design the new process so that it is chemically balanced and the pH remains at a steady state with no shift in acidity over time. It can therefore continue operating efficiently over long periods. In their tests, the system ran for over 200 hours with no significant decrease in output. The whole process can be done at ambient temperatures and relatively low pressures (about five times atmospheric pressure).
Another issue was that unwanted side reactions produced other chemical products that were not useful, but the team figured out a way to prevent these side reactions by the introduction of an extra “buffer” layer of bicarbonate-enriched fiberglass wool that blocked these reactions.
The team also built a fuel cell specifically optimised for the use of this formate fuel to produce electricity. The stored formate particles are simply dissolved in water and pumped into the fuel cell as needed. Although the solid fuel is much heavier than pure hydrogen, when the weight and volume of the high-pressure gas tanks needed to store hydrogen is considered, the end result is an electricity output near parity for a given storage volume, Li says.
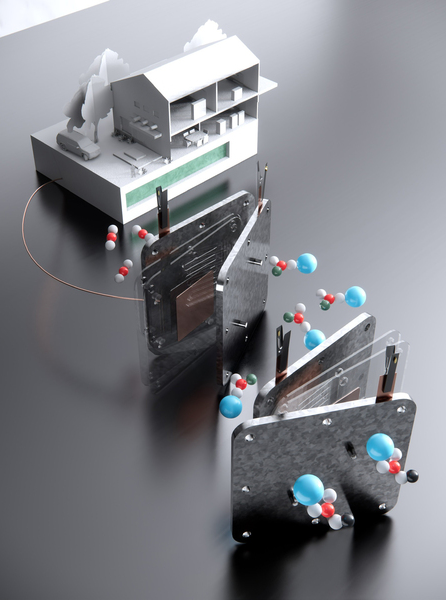
A schematic shows the formate process. The top left shows a household powered by the direct formate fuel cell, with formate fuel stored in the underground tank. In the middle, the fuel cell that harnesses formate to supply electricity is shown. On the lower right is the electrolyser that converts bicarbonate into formate. / IMAGE: Shuhan Miao, Harvard Graduate School of Design
Home-scale and grid-scale
The formate fuel can potentially be adapted for anything from home-sized units to large scale industrial uses or grid-scale storage systems, the researchers say. Initial household applications might involve an electrolyser unit about the size of a refrigerator to capture and convert the carbon dioxide into formate, which could be stored in an underground or rooftop tank. Then, when needed, the powdered solid would be mixed with water and fed into a fuel cell to provide power and heat. “This is for community or household demonstrations,” Zhang says, “but we believe that also in the future it may be good for factories or the grid.”
“The formate economy is an intriguing concept because metal formate salts are very benign and stable, and a compelling energy carrier,” says Ted Sargent, a professor of chemistry and of electrical and computer engineering at Northwestern University, who was not associated with this work. “The authors have demonstrated enhanced efficiency in liquid-to-liquid conversion from bicarbonate feedstock to formate, and have demonstrated these fuels can be used later to produce electricity,” he says.
The work was supported by the U.S. Department of Energy Office of Science.
***
David Chandler is an Institute Writer at MIT
Reprinted with permission of MIT News
