 In most lithium-ion batteries, the cathode contains cobalt. But cobalt is a scarce metal, found mostly in politically unstable countries, its extraction is hazardous for miners and generates toxic waste. And as demand for batteries globally keeps rising, so too will the cost of cobalt. Anne Trafton at MIT describes the development of an alternative cathode made of organic materials. Its structure is similar to graphite. It can conduct electricity like cobalt, and batteries made with it have comparable storage capacity, can be charged up faster, and can be produced at half the cost of the cobalt-containing batteries. What’s more, the primary materials needed to manufacture the new organic cathodes are already commercially available and produced in large quantities as commodity chemicals.
In most lithium-ion batteries, the cathode contains cobalt. But cobalt is a scarce metal, found mostly in politically unstable countries, its extraction is hazardous for miners and generates toxic waste. And as demand for batteries globally keeps rising, so too will the cost of cobalt. Anne Trafton at MIT describes the development of an alternative cathode made of organic materials. Its structure is similar to graphite. It can conduct electricity like cobalt, and batteries made with it have comparable storage capacity, can be charged up faster, and can be produced at half the cost of the cobalt-containing batteries. What’s more, the primary materials needed to manufacture the new organic cathodes are already commercially available and produced in large quantities as commodity chemicals.
Cobalt-free batteries could power cars of the future. MIT chemists developed a battery cathode based on organic materials, which could reduce the EV industry’s reliance on scarce metals. By Anne Trafton, MIT News.
A cathode based on organic materials
Many electric vehicles are powered by batteries that contain cobalt — a metal that carries high financial, environmental, and social costs.
MIT researchers have now designed a battery material that could offer a more sustainable way to power electric cars. The new lithium-ion battery includes a cathode based on organic materials, instead of cobalt or nickel (another metal often used in lithium-ion batteries).
In a new study, the researchers showed that this material, which could be produced at much lower cost than cobalt-containing batteries, can conduct electricity at similar rates as cobalt batteries. The new battery also has comparable storage capacity and can be charged up faster than cobalt batteries, the researchers report.
“I think this material could have a big impact because it works really well,” says Mircea Dincă, the W.M. Keck Professor of Energy at MIT. “It is already competitive with incumbent technologies, and it can save a lot of the cost and pain and environmental issues related to mining the metals that currently go into batteries.”
Dincă is the senior author of the study, which appears in the journal ACS Central Science. Tianyang Chen PhD ’23 and Harish Banda, a former MIT postdoc, are the lead authors of the paper. Other authors include Jiande Wang, an MIT postdoc; Julius Oppenheim, an MIT graduate student; and Alessandro Franceschi, a research fellow at the University of Bologna.
The multiple downsides of cobalt
Most electric cars are powered by lithium-ion batteries, a type of battery that is recharged when lithium ions flow from a positively charged electrode, called a cathode, to a negatively charged electrode, called an anode. In most lithium-ion batteries, the cathode contains cobalt, a metal that offers high stability and energy density.
However, cobalt has significant downsides. A scarce metal, its price can fluctuate dramatically, and much of the world’s cobalt deposits are located in politically unstable countries. Cobalt extraction creates hazardous working conditions and generates toxic waste that contaminates land, air, and water surrounding the mines.
“Cobalt batteries can store a lot of energy, and they have all of features that people care about in terms of performance, but they have the issue of not being widely available, and the cost fluctuates broadly with commodity prices. And, as you transition to a much higher proportion of electrified vehicles in the consumer market, it’s certainly going to get more expensive,” Dincă says.
Alternatives to cobalt
Because of the many drawbacks to cobalt, a great deal of research has gone into trying to develop alternative battery materials. One such material is lithium-iron-phosphate (LFP), which some car manufacturers are beginning to use in electric vehicles. Although still practically useful, LFP has only about half the energy density of cobalt and nickel batteries.
Another appealing option are organic materials, but so far most of these materials have not been able to match the conductivity, storage capacity, and lifetime of cobalt-containing batteries. Because of their low conductivity, such materials typically need to be mixed with binders such as polymers, which help them maintain a conductive network. These binders, which make up at least 50 percent of the overall material, bring down the battery’s storage capacity.
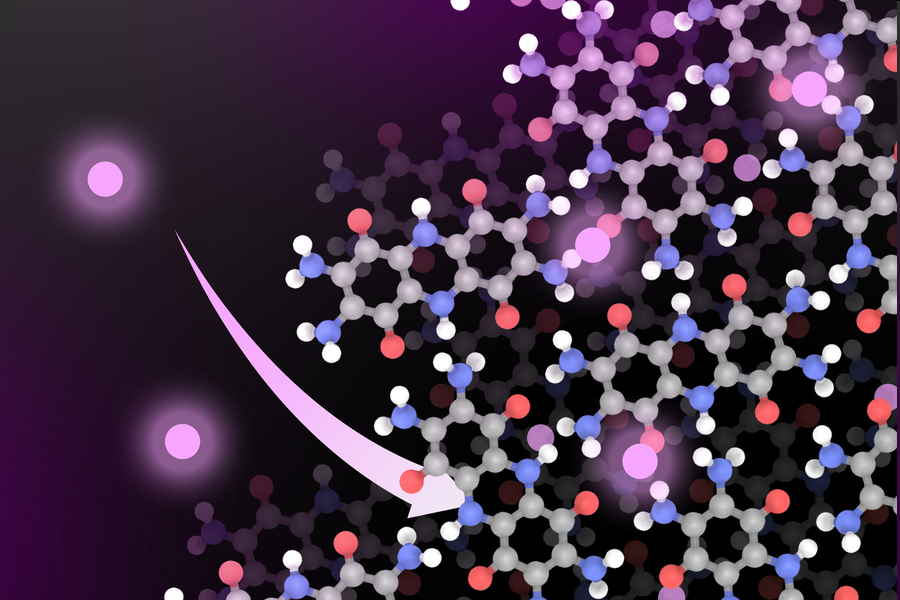
A new MIT battery material could offer a more sustainable way to power electric cars. Instead of cobalt or nickel, the new lithium-ion battery includes a cathode based on organic materials. In this image, lithium molecules are shown in glowing pink. / IMAGE: Courtesy of the researchers. Edited by MIT News.
An organic conductor
About six years ago, Dincă’s lab began working on a project, funded by Lamborghini, to develop an organic battery that could be used to power electric cars. While working on porous materials that were partly organic and partly inorganic, Dincă and his students realised that a fully organic material they had made appeared to show that it might be a strong conductor.
This material consists of many layers of TAQ (bis-tetraaminobenzoquinone), an organic small molecule that contains three fused hexagonal rings. These layers can extend outward in every direction, forming a structure similar to graphite. Within the molecules are chemical groups called quinones, which are the electron reservoirs, and amines, which help the material to form strong hydrogen bonds.
Those hydrogen bonds make the material highly stable and also very insoluble. That insolubility is important because it prevents the material from dissolving into the battery electrolyte, as some organic battery materials do, thereby extending its lifetime.
“One of the main methods of degradation for organic materials is that they simply dissolve into the battery electrolyte and cross over to the other side of the battery, essentially creating a short circuit. If you make the material completely insoluble, that process doesn’t happen, so we can go to over 2,000 charge cycles with minimal degradation,” Dincă says.
Strong performance
Tests of this material showed that its conductivity and storage capacity were comparable to that of traditional cobalt-containing batteries. Also, batteries with a TAQ cathode can be charged and discharged faster than existing batteries, which could speed up the charging rate for electric vehicles.
To stabilise the organic material and increase its ability to adhere to the battery’s current collector, which is made of copper or aluminium, the researchers added filler materials such as cellulose and rubber. These fillers make up less than one-tenth of the overall cathode composite, so they don’t significantly reduce the battery’s storage capacity.
These fillers also extend the lifetime of the battery cathode by preventing it from cracking when lithium ions flow into the cathode as the battery charges.
Half the cost of cobalt batteries
The primary materials needed to manufacture this type of cathode are a quinone precursor and an amine precursor, which are already commercially available and produced in large quantities as commodity chemicals. The researchers estimate that the material cost of assembling these organic batteries could be about one-third to one-half the cost of cobalt batteries.
Lamborghini has licensed the patent on the technology. Dincă’s lab plans to continue developing alternative battery materials and is exploring the possible replacement of lithium with sodium or magnesium, which are cheaper and more abundant than lithium.
***
Anne Trafton is a Senior Writer at MIT News
Reprinted with permission of MIT News
